Structure and properties of molybdenum oxide nitrides as model systems for selective oxidation catalysts.
Kühn S, Schmidt-Zhang P, Hahn AH, Huber M, Lerch M, Ressler T -Chemistry Central journal(2011)
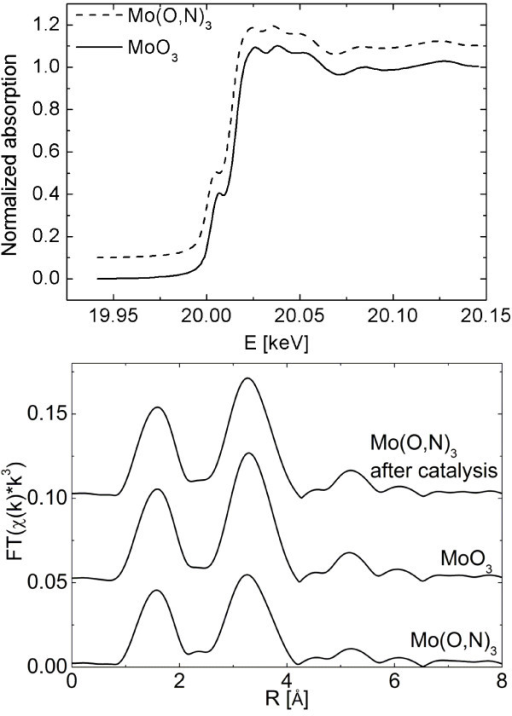
F2:XANES spectra of α-MoO3 and Mo(O,N)3 at 298 K (top) and Fourier transformed χ(k) of α-MoO3 and Mo(O,N)3 (before and after catalysis) (bottom).
View Article:PubMed Central - HTML - PubMed
Affiliation:Technische Universität Berlin, Institut für Chemie, Sekr, C2, Strasse des 17, Juni 135, D-10623 Berlin, Germany. thorsten.ressler@tu-berlin.de.
Additional Figures:
Article
Collection
Results
Bottom Line:Compared to regular α-MoO3, Mo(O,N)3 exhibited a higher electronic and ionic conductivity and an onset of reduction in propene at lower temperatures.Apparently, the increased reducibility, oxygen mobility, and conductivity of Mo(O,N)3 compared to α-MoO3 had no effect on the catalytic behavior of the two catalysts.The results presented confirm the suitability of molybdenum oxide nitrides as model systems for studying bulk contributions to selective oxidation.
Abstract
Molybdenum oxide nitride (denoted as Mo(O,N)3) was obtained by ammonolysis of α-MoO3 with gaseous ammonia. Electronic and geometric structure, reducibility, and conductivity of Mo(O,N)3 were investigated by XRD, XAS, UV-Vis spectroscopy, and impedance measurements. Catalytic performance in selective propene oxidation was determined by online mass spectrometry und gas chromatography. Upon incorporation of nitrogen, Mo(O,N)3 maintained the characteristic layer structure of α-MoO3. XRD analysis showed an increased structural disorder in the layers while nitrogen is removed from the lattice of Mo(O,N)3 at temperatures above ~600 K. Compared to regular α-MoO3, Mo(O,N)3 exhibited a higher electronic and ionic conductivity and an onset of reduction in propene at lower temperatures. Surprisingly, α-MoO3 and Mo(O,N)3 exhibited no detectable differences in onset temperatures of propene oxidation and catalytic selectivity or activity. Apparently, the increased reducibility, oxygen mobility, and conductivity of Mo(O,N)3 compared to α-MoO3 had no effect on the catalytic behavior of the two catalysts. The results presented confirm the suitability of molybdenum oxide nitrides as model systems for studying bulk contributions to selective oxidation.
Mentions
XRD patterns and XAS spectra of α-MoO3 and Mo(O,N)3 are shown in Figure 1 and Figure 2, respectively. The XRD pattern and the XANES and EXAFS spectra of Mo(O,N)3 are similar to those of α-MoO3 without additional phases detectable. Crystallite size of α-MoO3 amounted to ~ 60 nm as calculated from the integral breadths of XRD peaks using the Scherrer equation [15]. Apparently, the long-range order and short-range order structure of α-MoO3 is preserved after incorporation of nitrogen. Despite the overall agreement, subtle differences can be seen in the XRD patterns and XAS data of α-MoO3 and Mo(O,N)3. An increased XRD peak broadening (Figure 1) and a reduced XAFS amplitude (Figure 2) indicate an increased formation of defects in the Mo(O,N)3 structure upon nitridation. It is suggested that nitrogen atoms substitute for oxygen in the layers of α-MoO3 leading to an increased strain and a corresponding peak broadening in the XRD pattern. Similarly, an increased distance distribution around the Mo centers in Mo(O,N)3 resulted in the XAFS amplitude reduction observed. Moreover, analysis of the Mo K edge shift revealed an average valence of +5.86 for Mo(O,N)3 and +5.96 for the α-MoO3 used as reference [16]. According to the simplified defect model assumed incorporation of nitrogen results in formation of vacancies in the anion lattice (). Therefore, the slight reduction in Mo average valence should not be related to the presence of nitrogen but rather originate from a slight reduction of α-MoO3 by gaseous ammonia during sample preparation. However, the corresponding 5% MoO2 were not detectable in the XRD or XAFS data of Mo(O,N)3.
MeSH